Introduction-
We see and use a number of substances in our daily life. These are pure as well as impure mixture. Most of them are mixture. E.g.- Air, Water, Milk, etc.The separation of harmful or useless component is done from useful ones by different methods.
Pure Substance-
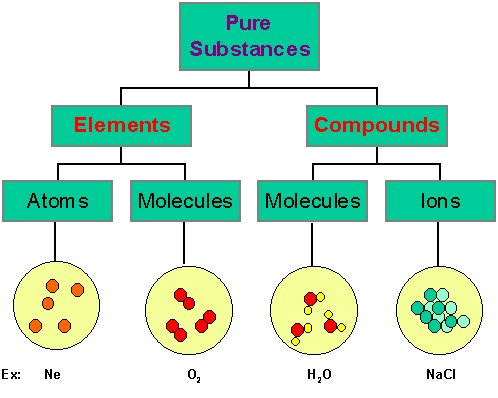
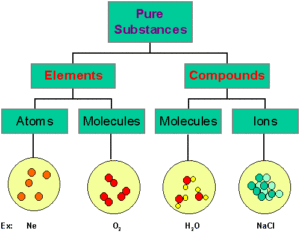
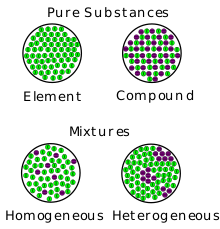
Those substances which are made up of only one kind of particles are called as pure substances. e.g.- All elements like Hydrogen, Gold, Carbon etc. and Compounds like Water, Sodium Chloride etc.
Mixture-
Those impure substances in which two or more other substances are found are called mixture.
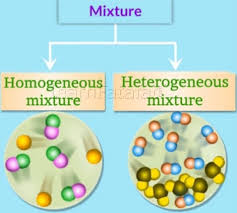
The ratio of components in the mixture is not fixed. Their individual chemical characteristic is retained. Components of a mixture can be separated. A mixture can be solid, liquid or gas. The mixtures can be –homogeneous or heterogeneous. The components of homogeneous mixture cannot be seen separately. e. g.- Steel, Petroleum, milk, Air etc. The components of heterogeneous mixture can be seen separately. e. g.- Rice and stone, sand and iron etc. etc.
Separation of substances-
The separation of substances is done to remove harmful component or unwanted component or to obtain pure or useful component from a mixture. The common methods are-
1. Hand Picking-The process of separating of large unwanted components of the mixture by hand is called handpicking. e. g.- Small pieces of stones from rice, pulses etc.
2. Threshing-The process of separating of grains from their straw and chaff is called threshing. It is done manually by hand or by thresher machines.
3. Winnowing-The process of separating of lighter components from heavier component by using wind is called winnowing. e. g. Husk (lighter) from the grains (heavier).
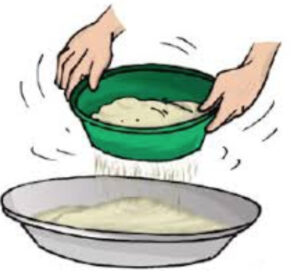
4. Sieving-The process of separating different size components of a mixture by using sieve is called sieving. e. g. Tea from tea leaves by sieve, Fine sand from gravels and pebbles of stone by using wire-mesh.
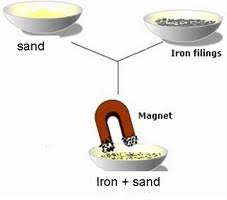
5. Magnetic separation-The process of separating of a non-magnetic component from a magnetic component of the given mixture is called magnetic separation.
e.g.- Separation of iron objects from scrap yard, Iron nails, from sand etc.
6. Sedimentation, Decantation and Filtration- The process of separating of insoluble solids from liquid in its solution is done by sedimentation, decantation and filtration. These are interrelated processes.
The heavier insoluble particles are allowed to settle down at the bottom of container. It is called sediment.
The pouring of liquid part without disturbing the sediment is called decantation.
The process by which very fine insoluble components are removed using filter/ filter paper is called filtration.
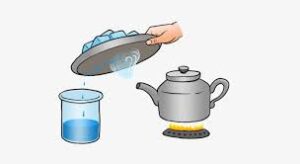
7. Evaporation and Condensation- The changing of water into water vapour is called evaporation or vaporization. Evaporation is used to separate water soluble solids from liquids.
Evaporation is used to make salt from salty water. The liquid evaporates and heavy solid component is left behind. Condensation is changing of water vapour into liquid form by cooling. The solid impurities are left behind on evaporation. Then pure liquid is obtained from this vapour by condensation.
8. Centrifugation or Churning-The fine insoluble solid is separated from liquid-solid mixture by rotating at very high speed is called centrifugation. e. g.- Butter from milk, drying of wet clothes by squeezing water in washing machine, blood cells from plasma etc.
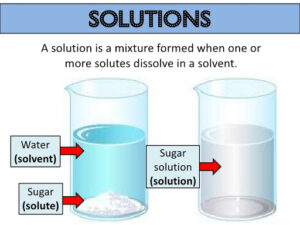
Saturated Solution-
The solute and solvent together is called as solution. e. g.- Sugar is a solute and water is a solvent that together forms a solution.
Solute (Sugar) + Solvent (Water) = Solution
If more solute is added in the solution it will become denser. A solution in which no more solute can be dissolved is called saturated solution. The solute will be seen more at the base of container.
On increasing temperature of saturated solution, more solutes can be added. On the other hand on decreasing the temperature of saturated solution more solutes cannot be added.
Solved Exercise Questions
Q.1 Why do we need to separate different components of a mixture? Give two examples.
Ans-We separate different components of a mixture for different purposes. These purposes may be-
(a) To remove harmful components from the mixture- If some unwanted harmful component is present in the mixture, it is separated from the useful valuable component. e. g.- Harmful salts are removed from water to make it fit for drinking.
(b) To get useful components from the mixture- In refining of crude oil (petroleum) different useful components can be separated by fractional distillation method. Similarly, in extraction of metals from their ores through magnetic separation method, the metals are separated from non-magnetic components.
Q.2 What is winnowing? Where is it used?
Ans-Winnowing is the process of separating heavier and lighter components of a mixture by wind or blowing air. Farmers separate grains from husk by the method of winnowing.
Q.3 How will you separate husk or dust particles from sample of sample of pulses before cooking?
Ans-The dirt particles that are present in the pulses are removed by washing the mixture with water. Being heavier, the pulses settle down, while the dirt particle being lighter keep floating on water . This process is known as sedimentation. The dirt water can be removed by method of decantation leaving the pulses at bottom
Q.4 What is sieving? Where it is used?
Ans-The process of separating different size components of a mixture by using sieve is called sieving. e. g. Tea from tea leaves by sieve, Fine sand from gravels and pebbles of stone by using wire-mesh. It is uses to separate useful substance from the unwanted substance.
Q.5 How will you separate sand and water from their mixture?
Ans. We will separate sand and water by sedimentation and decantation method. First we leave this mixture for some time. After some time, the sand which is; heavier get settled down at the bottom. After that we wall pour water into another container and the mixture will be separated.
Q.6 Is it possible to separate sugar mixed with wheat flour? If yes, how will you do it?
Ans. Sugar can be separated from wheat flour by sieving. Due to difference in the size of particles, sugar will stay on sieve and wheat flour will pass through it.
Q.7 How would you obtain clear water from a sample of muddy water?
Ans. The clear water can be obtained from a sample of muddy water by the process of filtration.
A filter paper is one such filter that has very fine pores in it. A filter paper folded in the form of a cone is fixed in a funnel. The mixture is then poured on the filter paper. Solid particles in the mixture do not pass through it and remain on the filter.
Q.8 Fill in the blanks:
(a) The method of separating seeds of paddy from its stalks is called …………….
(b) When milk, cooled after boiling, is poured onto a piece of cloth the cream (malai) is left behind on it. This process of separating cream from milk is an example of ………….. .
(a) Salt is obtained from sea water by the process of ………………
(b) Impurities settled at the bottom when muddy water was kept overnight in a bucket. The clear water was then poured off from the top. The process of separation used in this example is called ……………
Ans.
(a) threshing
(b)filtration
(b) evaporation
(d) sedimentation and decantation
Q.9 True or false?
(a) A mixture of milk and water can be separated by filtration.
(b) A mixture of powdered salt and sugar can be separated by the process of winnowing.
(c) Separation of sugar from tea can be done with filtration.
(d) Grain and husk can be separated with the process of decantation.
Ans.
(a) False
(b) False
(c) False
(d) False
Q.10 Lemonade is prepared by mixing lemon juice and sugar in water. You wish to add ice to cool it. Should you add ice to the lemonade before or after dissolving sugar? In which case would it be possible to dissolve more sugar?
Ans. We should add ice after dissolving sugar. When the temperature is high then more sugar can be dissolved. After mixing ice it gets cool and less sugar will dissolve in it.
© www.vkscience.com








Tq sir
Welcome
Tq sir