<script async src="https://pagead2.googlesyndication.com/pagead/js/adsbygoogle.js?client=ca-pub-7606197531762322"
crossorigin="anonymous"></script>
Introduction-Current is the flow of charges through a conducting wire or liquid. Those materials through which current can flow are called as conductors. e. g- Metal wires, Conducting liquids having electrolyte, . Those materials through which current cannot flow are called as poor conductors or insulators. e. g- Plastic, Dry wood, Paper, Distilled water, Edible oils, like Mustard oil, Coconut oil, Milk, Honey etc.
Do liquids conduct electricity?– Whether the liquid will conduct the current of not it depends on the minerals –salts dissolved in it. The mineral salts, acids and bases act as conductor of current in water or any similar liquid.
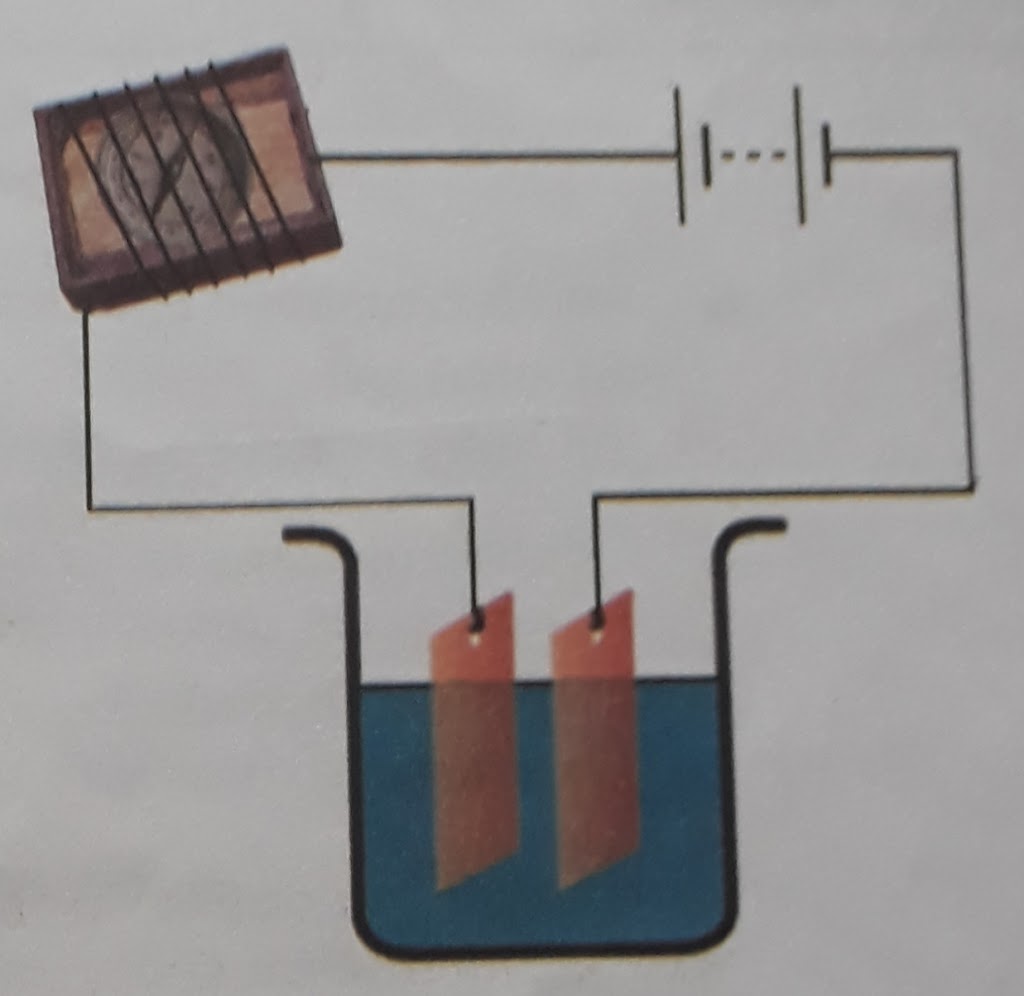
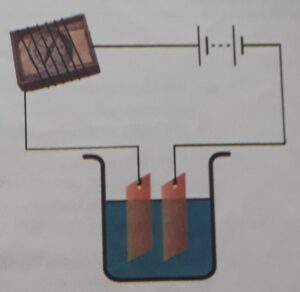
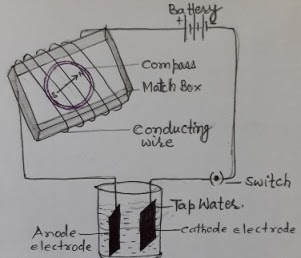
A simple electric circuit was prepared using battery, connecting wire, switch and magnetic compass. The compass was kept in a match box and connecting wire is wrapped around it. The two open ends of connecting wires were kept in a beaker having tap water. When current passes through the circuit, defection of needle of magnetic compass was seen. It showed that tap water is a conductor of current. Similarly, when beaker of tap water was replaced by distilled water, there was no deflection in the compass. It showed that distilled water is an insulator of current owing to the lack of mineral-salts.
|
S.N. |
MATERIAL |
DEFLECTION IN COMPASS NEEDLE (YES/NO) |
GOOD /POOR CONDUCTOR |
|
1 |
Tap Water |
Yes |
Good Conductor |
|
2 |
Milk |
Yes |
Good Conductor |
|
3 |
Mustard Oil |
No |
Poor Conductor |
|
4 |
Vinegar |
Yes |
Good Conductor |
|
5 |
Lemon Juice |
Yes |
Good Conductor |
|
6 |
Honey |
No |
Poor Conductor |
|
7 |
Distilled Water |
No |
Poor Conductor |
|
8 |
Salt+ Distilled Water |
Yes |
Good Conductor |
LED-(Light Emitting Diodes) – The smaller L.E.Ds glow even at very less current of 0.3 V. They are available in form of bulb and tube lights also. They consume comparatively less current than bulbs and C.F.Ls. There are two wires attached to L.E.Ds called leads. One lead is longer than the other. Loner one is connected with positive terminal of the battery while smaller one is connected with negative terminal of the battery.
Chemical Effects of electric current- When current is allowed to passes through any solution, either current passes through it or not. The solutions behave as conductors when mineral-salts remain dissolved in them. Sometimes when an electrolyte (that dissociates into ions on passing current) is dissolved as solute, then also the solution behaves as a conductor. Ions are charged particles that are formed on dissociation of an electrolyte when current passes in its solution.
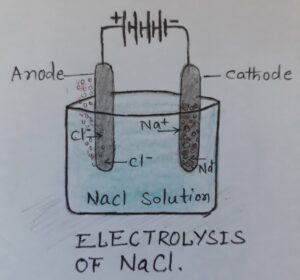
e.g.- The sodium chloride (common edible salt) is an electrolyte that dissociates into its ions on passing the current in its solution.
NaCl (Sodium Chloride) ———–> Na + (Sodium Ion) + Cl – (Chlorine Ion)
The positively charged ion is called cation and negatively charged ion is called as anion. This breaking of any electrolytic compound into their constituent ions is called as electrolysis. This happens when there are two plates in the electrolytic cell, connected to the positive and negative terminal of the battery. The two metal plates are called as electrodes- Cathode and Anode. Cathode remains connected with negative terminal of the battery and anode remain connected to the positive terminal of the battery. During the electrolysis the cations go to cathode and anions go to anode electrode/terminal. Electrolysis is a chemical effect of electric current.
Electrolytes are solutions of acids, bases and salts. The ions help in carrying the charges.
Electroplating- The electrolysis has many scientific and industrial applications. One of the important applications is electroplating. Electroplating is a process of coating of a layer of desired metal on another material by means of electric current. Electroplating is done to protect the object from rusting, scratching or to make it more attractive.
The strip of metal whose coating is to be done is taken as anode. The metal to be electroplated is taken as cathode. The electrolyte is taken as the soluble salt of metal hose coating is to be done at anode. Generally, nickel, gold, silver, chromium, platinum like metals are coated over a relatively less expensive metal object. The proper cleaning of metal object to be electroplated and supply of current of suitable strength is essential for long lasting electroplating.
Exercise Questions-
Q.1 Fill in the blanks
(a) Most liquids that conduct electricity are solutions of ………..and ……………
(b) The passage of an electric current through a solution causes ……………….. effects.
(c) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the …………………..terminal of the battery.
(d) The process of depositing a layer of any desired metal by means of electricity is called …………..
Ans- (a) acids, bases (alkali) and salts (b) chemical (c) negative (d) electroplating
Q.2 When the free end of a tester is dipped into a solution, the magnetic needle shows deflection. Can you explain the reason?
A
Electro-refining- The metals are extracted from their ores. For extraction of metal, such metal ores are taken in which metal is found in comparatively maximum percentage.
<script async src=”https://pagead2.googlesyndication.com/pagead/js/adsbygoogle.js?client=ca-pub-7606197531762322″
crossorigin=”anonymous”></script>